Active Pharmaceutical Ingredient (API) Market Trends, Growth, Size and Forecast 2030
Active Pharmaceutical Ingredient (API) Market Size, Share & Trends Analysis By Type, By Manufacturer, By types of Synthesis, By type of Drug, By Therapeutic Application, Regional Outlook, Competitive Strategies and Segment Forecasts to 2030
| Published: Feb-2022 | Report ID: PHAR2202 | Pages: 1 - 207 | Formats*: |
| Category : Pharmaceutical | |||
| Report Metric | Details |
| Market size available for years | 2019-2030 |
| Base year considered | 2021 |
| Forecast period | 2022-2030 |
| Segments covered | By type, By Manufacturer, By types of Synthesis, By types of Drug, By types of Therapeutic action, By Region |
| Geographies covered | North America, Europe, Asia, and Rest of the World |
| Companies Covered | Pfizer, Inc. (US), Novartis International AG (Switzerland), Sanofi (France), Boehringer Ingelheim (Germany), Bristol-Myers Squibb (US), Teva Pharmaceutical Industries Ltd. (Israel), Eli Lilly and Company (US), GlaxoSmithKline plc (UK), Merck & Co., Inc. (US), AbbVie Inc. (US), F. Hoffmann-La Roche Ltd. (Switzerland), AstraZeneca (UK), Cipla, Inc. (India), Mylan N.V. (US), Dr. Reddy’s Laboratories Ltd. (India), Sun Pharmaceutical Industries Ltd. (India), API Pharma Tech (India), BDR Pharmaceuticals Internationals Pvt. Ltd. (India), Sreepathi Pharmaceuticals Limited (India), Shilpa Medicare Limited (India) |
4.1. Introduction4.2. Market Dynamics
4.2.1. Drivers4.2.2. Restraints4.2.3. Opportunities4.2.4. Challenges
4.3. COVID-19 Impact on the Active Pharmaceutical Ingredient (API) Market4.4. Market Trends
5.1. Innovative APIs5.2. Generic APIS
6.1. Captive Manufacturers
7.1.1. Merchant Manufacturers Market, by Type7.1.2. Innovative Merchant API Manufacturers7.1.3. Generic Merchant API Manufacturers7.1.4. Merchant Manufacturers Market, by Type of Synthesis7.1.5. Synthetic APIs7.1.6. Biotech APIs
8.1. Synthetic APIs Market
8.1.1. Synthetic APIs Market, by Type8.1.2. Innovative Synthetic APIs8.1.3. Generic Synthetic APIs
8.2. Biotech APIs Market
8.2.1. Biotech APIs Market, by Type8.2.2. Innovative Biotech APIs8.2.3. Biosimilars8.2.4. Biotech APIs Market, by Product8.2.5. Monoclonal Antibodies8.2.6. Hormones8.2.7. Cytokines8.2.8. Fusion Proteins8.2.9. Therapeutic Enzymes8.2.10. Vaccines8.2.11. Blood Factors8.2.12. Biotech APIs Market, by Expression System8.2.13. Mammalian Expression Systems8.2.14. Microbial Expression Systems8.2.15. Yeast Expression Systems8.2.16. Insect Expression Systems8.2.17. Other Expression Systems (Plant expression systems)
9.1. Prescription Drugs9.2. Over-the-counter Drugs
10.1. Communicable Diseases10.2. Oncology10.3. Diabetes10.4. Cardiovascular Disease10.5. Pain management10.6. Respiratory Diseases10.7. Other Therapeutic Applications (Orthopedics, Urology and Nephrology, Ophthalmology, Pulmonology, Women’s Health, and Gastroenterology)
11.1. North America
11.1.1. US11.1.2. Canada
11.2. Europe
11.2.1. Germany11.2.2. France11.2.3. UK11.2.4. Italy11.2.5. Spain11.2.6. Hungary11.2.7. Rest of Europe
11.3. Asia
11.3.1. Japan11.3.2. China11.3.3. India11.3.4. South Korea11.3.5. Rest of Asia
11.4. Rest of the World11.5. Israel11.6. Other countries
12.1. Introduction12.2. Market Share Analysis, By Key Players12.3. Competitive Scenario
12.3.1. Product Launches12.3.2. Partnerships, Collaborations and Agreements12.3.3. Acquisitions12.3.4. Expansions12.3.5. Other Developments
13.1. AbbVie Inc. (US)13.2. API Pharma Tech (India)13.3. AstraZeneca (UK)13.4. BDR Pharmaceuticals Internationals Pvt. Ltd. (India)13.5. Boehringer Ingelheim (Germany)13.6. Bristol-Myers Squibb (US)13.7. Cipla, Inc. (India)13.8. Dr. Reddy’s Laboratories Ltd. (India)13.9. Eli Lilly and Company (US)13.10. F. Hoffmann-La Roche Ltd. (Switzerland)13.11. GlaxoSmithKline plc (UK)13.12. Merck & Co., Inc. (US)13.13. Mylan N.V. (US)13.14. Novartis International AG (Switzerland)13.15. Pfizer, Inc. (US)13.16. Sanofi (France)13.17. Shilpa Medicare Limited (India)13.18. Sreepathi Pharmaceuticals Limited (India)13.19. Sun Pharmaceutical Industries Ltd. (India)13.20. Teva Pharmaceutical Industries Ltd. (Israel)
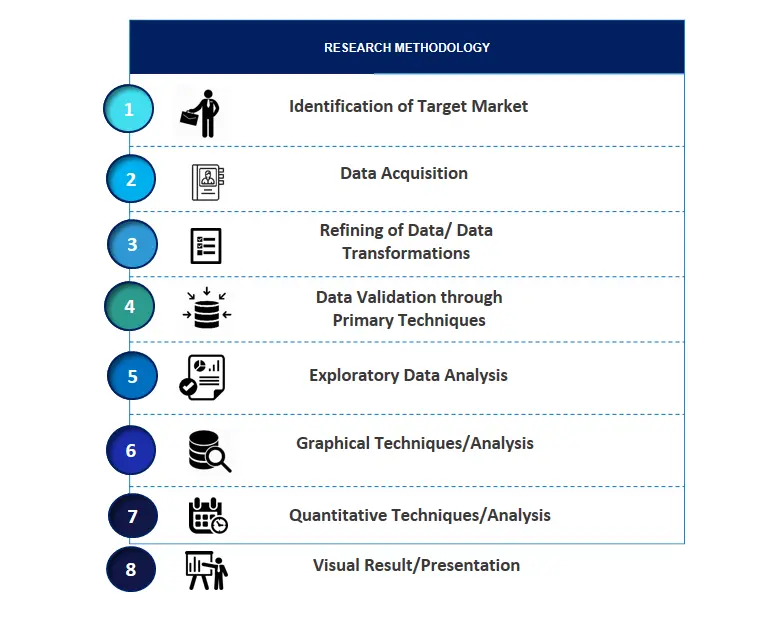
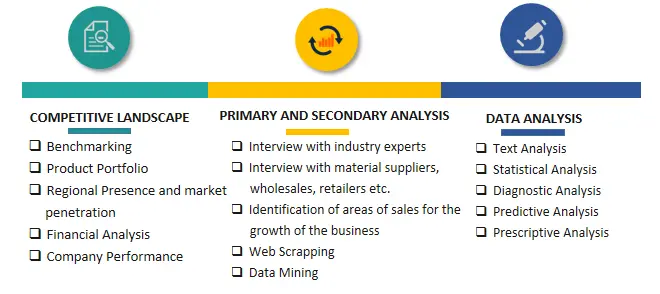
SPER Market Research’s methodology uses great emphasis on primary research to ensure that the market intelligence insights are up to date, reliable and accurate. Primary interviews are done with players involved in each phase of a supply chain to analyze the market forecasting. The secondary research method is used to help you fully understand how the future markets and the spending patterns look likes.
The report is based on in-depth qualitative and quantitative analysis of the Product Market. The quantitative analysis involves the application of various projection and sampling techniques. The qualitative analysis involves primary interviews, surveys, and vendor briefings. The data gathered as a result of these processes are validated through experts opinion. Our research methodology entails an ideal mixture of primary and secondary initiatives.
Frequently Asked Questions About This Report
PLACE AN ORDER
Year End Discount
Sample Report
Pre-Purchase Inquiry
NEED CUSTOMIZATION?
Request CustomizationCALL OR EMAIL US
100% Secure Payment






Related Reports
Our Global Clients
Our data-driven insights have influenced the strategy of 200+ reputed companies across the globe.