Global In-Vitro Diagnostics Market Insights, Size & Growth Forecast To 2028
Global In-Vitro Diagnostics Market - By Product Type (Reagents, Instruments, and Software & Services), By Technique (Immunodiagnostics, Hematology, Molecular Diagnostics, Tissue Diagnostics, Clinical Chemistry, and Other IVD Techniques), By Application (Infectious Diseases, Cancer, Cardiac Diseases, Immune System Disorders, Nephrological Diseases, Gastrointestinal Diseases, and Other Indications), and By End User (Standalone Laboratory, Hospitals, Academic & Medical Schools, Point of Care Testing, and Others)and By Region (North America, Europe, Asia Pacific, South America, and Middle East, & Africa)- Global forecast from 2021-2028
| Published: Apr-2021 | Report ID: HLCA2105 | Pages: 1 - 218 | Formats*: |
| Category : Healthcare | |||
- Abbott Laboratories
- Becton
- Dickinson and Company
- bioMérieux SA
- Bio-Rad Laboratories Inc.
- Danaher Corporation (Beckman Coulter, Inc.)
- F. Hoffmann-La Roche Ltd.
- Johnson & Johnson
- QIAGEN N.V.
- Sysmex Corporation
- Thermo Fisher Scientific Inc.
- and others are key players in the global In-Vitro Diagnostics market.
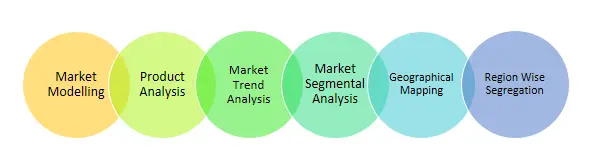
1.1. Market Modelling1.2. Product Analysis1.3. Market Trend and Economic Factors Analysis1.4. Market Segmental Analysis1.5. Geographical Mapping1.6. Country Wise Segregation
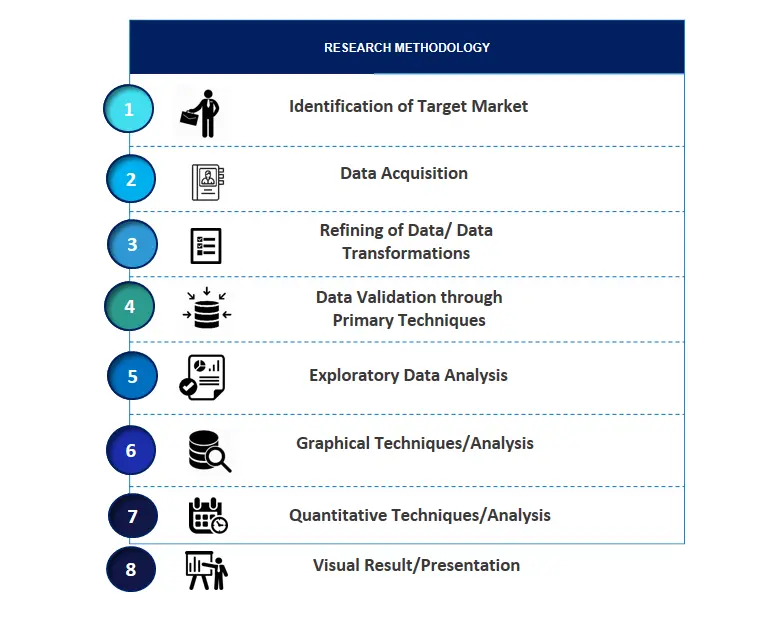
2.1. Identification of Target Market2.2. Data Acquisition2.3. Refining of Data/ Data Transformations2.4. Data Validation through Primary Techniques2.5. Exploratory Data Analysis2.6. Graphical Techniques/Analysis2.7. Quantitative Techniques/Analysis2.8. Visual Result/Presentation
4.1. Economic Factor Analysis
4.1.1. Drivers4.1.2. Trends4.1.3. Opportunities4.1.4. Challenges
4.2. Competitors & Product Analysis4.3. Regulatory Framework4.4. Company market share analysis, 20204.5. Porter’s Five forces analysis4.6. New Investment Analysis4.7. PESTEL Analysis
5.1. Market Size & Forecast, 2017-2028
5.1.1. Demand5.1.1.1. By Value (USD Million)
5.2. Market Share & Forecast, 2017-2028
5.2.1. By Product Type5.2.1.1. Reagents5.2.1.2. Instruments5.2.1.3. Software & Services5.2.2. By Technique5.2.2.1. Immunodiagnostics5.2.2.1.1.1. Enzyme-Linked Immunosorbent Assay (ELISA)5.2.2.1.1.2. Chemiluminescence Immunoassays (CLIAS)5.2.2.1.1.3. Fluorescence Immunoassays (FIAS)5.2.2.1.1.4. Colorimetric Immunoassays (CIS)5.2.2.2. Rapid Tests5.2.2.3. Enzyme-Linked ImmunoSpot Assays (ELISPOT)5.2.2.4. Radioimmunoassay5.2.2.5. Western Blot5.2.2.6. Hematology5.2.2.7. Molecular Diagnostics5.2.2.7.1.1. Polymerase Chain Reaction (PCR)5.2.2.7.1.2. Isothermal Nucleic Acid Amplification Technology (INAAT)5.2.2.7.1.3. Hybridization5.2.2.7.1.4. DNA Diagnostics5.2.2.7.1.5. Microarrays5.2.2.7.1.6. Others5.2.2.8. Tissue Diagnostics5.2.2.9. Clinical Chemistry5.2.2.9.1.1. Basic Metabolic Panel5.2.2.9.1.2. Liver Panel5.2.2.9.1.3. Lipid Profile5.2.2.9.1.4. Thyroid Function Panel5.2.2.9.1.5. Renal Profile5.2.2.9.1.6. Electrolyte Panel5.2.2.9.1.7. Specialty Chemicals5.2.2.10. Other IVD Techniques5.2.3. By Application5.2.3.1. Infectious Diseases5.2.3.2. Cancer5.2.3.3. Cardiac Diseases5.2.3.4. Immune System Disorders5.2.3.5. Nephrological Diseases5.2.3.6. Gastrointestinal Diseases5.2.3.7. Other Indications5.2.4. By End-user5.2.4.1. Standalone Laboratory5.2.4.2. Hospitals5.2.4.3. Academic & Medical Schools5.2.4.4. Point of Care Testing5.2.4.5. Others5.2.5. By Region5.2.5.1. Europe5.2.5.2. North America5.2.5.3. Asia Pacific5.2.5.4. South America5.2.5.5. Middle East & Africa
6.1. Europe In-Vitro Diagnostics Size & Forecast, 2017-2028
6.1.1. Demand6.1.1.1. By Value (USD Million)
6.2. Europe In-Vitro Diagnostics Market Share & Forecast, 2017-2028
6.2.1. By Product Type6.2.1.1. Reagents6.2.1.2. Instruments6.2.1.3. Software & Services6.2.2. By Technique6.2.2.1. Immunodiagnostics6.2.2.1.1.1. Enzyme-Linked Immunosorbent Assay (ELISA)6.2.2.1.1.2. Chemiluminescence Immunoassays (CLIAS)6.2.2.1.1.3. Fluorescence Immunoassays (FIAS)6.2.2.1.1.4. Colorimetric Immunoassays (CIS)6.2.2.2. Rapid Tests6.2.2.3. Enzyme-Linked ImmunoSpot Assays (ELISPOT)6.2.2.4. Radioimmunoassay6.2.2.5. Western Blot6.2.2.6. Hematology6.2.2.7. Molecular Diagnostics6.2.2.7.1.1. Polymerase Chain Reaction (PCR)6.2.2.7.1.2. Isothermal Nucleic Acid Amplification Technology (INAAT)6.2.2.7.1.3. Hybridization6.2.2.7.1.4. DNA Diagnostics6.2.2.7.1.5. Microarrays6.2.2.7.1.6. Others6.2.2.8. Tissue Diagnostics6.2.2.9. Clinical Chemistry6.2.2.9.1.1. Basic Metabolic Panel6.2.2.9.1.2. Liver Panel6.2.2.9.1.3. Lipid Profile6.2.2.9.1.4. Thyroid Function Panel6.2.2.9.1.5. Renal Profile6.2.2.9.1.6. Electrolyte Panel6.2.2.9.1.7. Specialty Chemicals6.2.2.10. Other IVD Techniques6.2.3. By Application6.2.3.1. Infectious Diseases6.2.3.2. Cancer6.2.3.3. Cardiac Diseases6.2.3.4. Immune System Disorders6.2.3.5. Nephrological Diseases6.2.3.6. Gastrointestinal Diseases6.2.3.7. Other Indications6.2.4. By End-user6.2.4.1. Standalone Laboratory6.2.4.2. Hospitals6.2.4.3. Academic & Medical Schools6.2.4.4. Point of Care Testing6.2.4.5. Others6.2.5. By Country6.2.5.1. Germany6.2.5.2. UK6.2.5.3. France6.2.5.4. Italy6.2.5.5. Rest of Europe6.2.6. Company Market Share (Top 3-5)6.2.7. Economic Impact Study on Europe In-Vitro Diagnostics Market
7.1. North America In-Vitro Diagnostics Market Size & Forecast, 2017-2028
7.1.1. Demand7.1.1.1. By Value (USD Million)
7.2. North America In-Vitro Diagnostics Market Share & Forecast, 2017-2028
7.2.1. By Product Type7.2.1.1. Reagents7.2.1.2. Instruments7.2.1.3. Software & Services7.2.2. By Technique7.2.2.1. Immunodiagnostics7.2.2.1.1.1. Enzyme-Linked Immunosorbent Assay (ELISA)7.2.2.1.1.2. Chemiluminescence Immunoassays (CLIAS)7.2.2.1.1.3. Fluorescence Immunoassays (FIAS)7.2.2.1.1.4. Colorimetric Immunoassays (CIS)7.2.2.2. Rapid Tests7.2.2.3. Enzyme-Linked ImmunoSpot Assays (ELISPOT)7.2.2.4. Radioimmunoassay7.2.2.5. Western Blot7.2.2.6. Hematology7.2.2.7. Molecular Diagnostics7.2.2.7.1.1. Polymerase Chain Reaction (PCR)7.2.2.7.1.2. Isothermal Nucleic Acid Amplification Technology (INAAT)7.2.2.7.1.3. Hybridization7.2.2.7.1.4. DNA Diagnostics7.2.2.7.1.5. Microarrays7.2.2.7.1.6. Others7.2.2.8. Tissue Diagnostics7.2.2.9. Clinical Chemistry7.2.2.9.1.1. Basic Metabolic Panel7.2.2.9.1.2. Liver Panel7.2.2.9.1.3. Lipid Profile7.2.2.9.1.4. Thyroid Function Panel7.2.2.9.1.5. Renal Profile7.2.2.9.1.6. Electrolyte Panel7.2.2.9.1.7. Specialty Chemicals7.2.2.10. Other IVD Techniques7.2.3. By Application7.2.3.1. Infectious Diseases7.2.3.2. Cancer7.2.3.3. Cardiac Diseases7.2.3.4. Immune System Disorders7.2.3.5. Nephrological Diseases7.2.3.6. Gastrointestinal Diseases7.2.3.7. Other Indications7.2.4. By End-user7.2.4.1. Standalone Laboratory7.2.4.2. Hospitals7.2.4.3. Academic & Medical Schools7.2.4.4. Point of Care Testing7.2.4.5. Others7.2.5. By Country7.2.5.1. US7.2.5.2. Canada7.2.5.3. Mexico7.2.6. Company Market Share (Top 3-5)7.2.7. Economic Impact Study on North America In-Vitro Diagnostics Market
8.1. Asia Pacific In-Vitro Diagnostics Market Size & Forecast, 2017-2028
8.1.1. Demand8.1.1.1. By Value (USD Million)
8.2. Asia Pacific In-Vitro Diagnostics Market Share & Forecast, 2017-2028
8.2.1. By Product Type8.2.1.1. Reagents8.2.1.2. Instruments8.2.1.3. Software & Services8.2.2. By Technique8.2.2.1. Immunodiagnostics8.2.2.1.1.1. Enzyme-Linked Immunosorbent Assay (ELISA)8.2.2.1.1.2. Chemiluminescence Immunoassays (CLIAS)8.2.2.1.1.3. Fluorescence Immunoassays (FIAS)8.2.2.1.1.4. Colorimetric Immunoassays (CIS)8.2.2.2. Rapid Tests8.2.2.3. Enzyme-Linked ImmunoSpot Assays (ELISPOT)8.2.2.4. Radioimmunoassay8.2.2.5. Western Blot8.2.2.6. Hematology8.2.2.7. Molecular Diagnostics8.2.2.7.1.1. Polymerase Chain Reaction (PCR)8.2.2.7.1.2. Isothermal Nucleic Acid Amplification Technology (INAAT)8.2.2.7.1.3. Hybridization8.2.2.7.1.4. DNA Diagnostics8.2.2.7.1.5. Microarrays8.2.2.7.1.6. Others8.2.2.8. Tissue Diagnostics8.2.2.9. Clinical Chemistry8.2.2.9.1.1. Basic Metabolic Panel8.2.2.9.1.2. Liver Panel8.2.2.9.1.3. Lipid Profile8.2.2.9.1.4. Thyroid Function Panel8.2.2.9.1.5. Renal Profile8.2.2.9.1.6. Electrolyte Panel8.2.2.9.1.7. Specialty Chemicals8.2.2.10. Other IVD Techniques8.2.3. By Application8.2.3.1. Infectious Diseases8.2.3.2. Cancer8.2.3.3. Cardiac Diseases8.2.3.4. Immune System Disorders8.2.3.5. Nephrological Diseases8.2.3.6. Gastrointestinal Diseases8.2.3.7. Other Indications8.2.4. By End-user8.2.4.1. Standalone Laboratory8.2.4.2. Hospitals8.2.4.3. Academic & Medical Schools8.2.4.4. Point of Care Testing8.2.4.5. Others8.2.5. By Country8.2.5.1. China8.2.5.2. India8.2.5.3. Japan8.2.5.4. Australia8.2.5.5. Rest of Asia Pacific8.2.6. Company Market Share (Top 3-5)8.2.7. Economic Impact Study on Asia Pacific In-Vitro Diagnostics Market
9.1. South America In-Vitro Diagnostics Market Size & Forecast, 2017-2028
9.1.1. Demand9.1.1.1. By Value (USD Million)
9.2. South America In-Vitro Diagnostics Market Share & Forecast, 2017-2028
9.2.1. By Product Type9.2.1.1. Reagents9.2.1.2. Instruments9.2.1.3. Software & Services9.2.2. By Technique9.2.2.1. Immunodiagnostics9.2.2.1.1.1. Enzyme-Linked Immunosorbent Assay (ELISA)9.2.2.1.1.2. Chemiluminescence Immunoassays (CLIAS)9.2.2.1.1.3. Fluorescence Immunoassays (FIAS)9.2.2.1.1.4. Colorimetric Immunoassays (CIS)9.2.2.2. Rapid Tests9.2.2.3. Enzyme-Linked ImmunoSpot Assays (ELISPOT)9.2.2.4. Radioimmunoassay9.2.2.5. Western Blot9.2.2.6. Hematology9.2.2.7. Molecular Diagnostics9.2.2.7.1.1. Polymerase Chain Reaction (PCR)9.2.2.7.1.2. Isothermal Nucleic Acid Amplification Technology (INAAT)9.2.2.7.1.3. Hybridization9.2.2.7.1.4. DNA Diagnostics9.2.2.7.1.5. Microarrays9.2.2.7.1.6. Others9.2.2.8. Tissue Diagnostics9.2.2.9. Clinical Chemistry9.2.2.9.1.1. Basic Metabolic Panel9.2.2.9.1.2. Liver Panel9.2.2.9.1.3. Lipid Profile9.2.2.9.1.4. Thyroid Function Panel9.2.2.9.1.5. Renal Profile9.2.2.9.1.6. Electrolyte Panel9.2.2.9.1.7. Specialty Chemicals9.2.2.10. Other IVD Techniques9.2.3. By Application9.2.3.1. Infectious Diseases9.2.3.2. Cancer9.2.3.3. Cardiac Diseases9.2.3.4. Immune System Disorders9.2.3.5. Nephrological Diseases9.2.3.6. Gastrointestinal Diseases9.2.3.7. Other Indications9.2.4. By End-user9.2.4.1. Standalone Laboratory9.2.4.2. Hospitals9.2.4.3. Academic & Medical Schools9.2.4.4. Point of Care Testing9.2.4.5. Others9.2.5. By Country9.2.5.1. Brazil9.2.5.2. Argentina9.2.5.3. Rest of South America9.2.6. Company Market Share (Top 3-5)9.2.7. Economic Impact Study on South America In-Vitro Diagnostics Market
10.1. Middle East & Africa In-Vitro Diagnostics Market Size & Forecast, 2017-2028
10.1.1. Demand10.1.1.1. By Value (USD Million)
10.2. Middle East & Africa In-Vitro Diagnostics Market Share & Forecast, 2018-2028
10.2.1. By Product Type10.2.1.1. Reagents10.2.1.2. Instruments10.2.1.3. Software & Services10.2.2. By Technique10.2.2.1. Immunodiagnostics10.2.2.1.1.1. Enzyme-Linked Immunosorbent Assay (ELISA)10.2.2.1.1.2. Chemiluminescence Immunoassays (CLIAS)10.2.2.1.1.3. Fluorescence Immunoassays (FIAS)10.2.2.1.1.4. Colorimetric Immunoassays (CIS)10.2.2.2. Rapid Tests10.2.2.3. Enzyme-Linked ImmunoSpot Assays (ELISPOT)10.2.2.4. Radioimmunoassay10.2.2.5. Western Blot10.2.2.6. Hematology10.2.2.7. Molecular Diagnostics10.2.2.7.1.1. Polymerase Chain Reaction (PCR)10.2.2.7.1.2. Isothermal Nucleic Acid Amplification Technology (INAAT)10.2.2.7.1.3. Hybridization10.2.2.7.1.4. DNA Diagnostics10.2.2.7.1.5. Microarrays10.2.2.7.1.6. Others10.2.2.8. Tissue Diagnostics10.2.2.9. Clinical Chemistry10.2.2.9.1.1. Basic Metabolic Panel10.2.2.9.1.2. Liver Panel10.2.2.9.1.3. Lipid Profile10.2.2.9.1.4. Thyroid Function Panel10.2.2.9.1.5. Renal Profile10.2.2.9.1.6. Electrolyte Panel10.2.2.9.1.7. Specialty Chemicals10.2.2.10. Other IVD Techniques10.2.3. By Application10.2.3.1. Infectious Diseases10.2.3.2. Cancer10.2.3.3. Cardiac Diseases10.2.3.4. Immune System Disorders10.2.3.5. Nephrological Diseases10.2.3.6. Gastrointestinal Diseases10.2.3.7. Other Indications10.2.4. By End-user10.2.4.1. Standalone Laboratory10.2.4.2. Hospitals10.2.4.3. Academic & Medical Schools10.2.4.4. Point of Care Testing10.2.4.5. Others10.2.5. By Country10.2.5.1. Saudi Arabia10.2.5.2. UAE10.2.5.3. South Africa10.2.5.4. Rest of Middle East & Africa10.2.6. Company Market Share (Top 3-5)10.2.7. Economic Impact Study on Middle East & Africa In-Vitro Diagnostics Market
11.1. Company Description11.2. Financial Analysis11.3. Key Products11.4. Key Management Personnel11.5. Contact Address11.6. SWOT Analysis11.7. Company Profile
11.7.1.1. Abbott Laboratories11.7.1.2. Becton, Dickinson and Company11.7.1.3. bioMérieux SA11.7.1.4. Bio-Rad Laboratories, Inc.11.7.1.5. Danaher Corporation (Beckman Coulter, Inc.)11.7.1.6. F. Hoffmann-La Roche Ltd.11.7.1.7. Johnson & Johnson11.7.1.8. QIAGEN N.V.11.7.1.9. Sysmex Corporation11.7.1.10. Thermo Fisher Scientific, Inc11.7.1.11. Other players
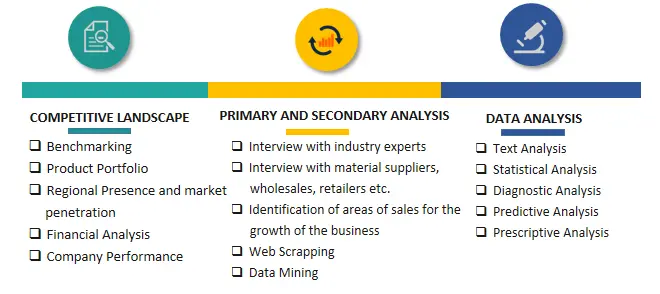
SPER Market Research’s methodology uses great emphasis on primary research to ensure that the market intelligence insights are up to date, reliable and accurate. Primary interviews are done with players involved in each phase of a supply chain to analyze the market forecasting. The secondary research method is used to help you fully understand how the future markets and the spending patterns look likes.
The report is based on in-depth qualitative and quantitative analysis of the Product Market. The quantitative analysis involves the application of various projection and sampling techniques. The qualitative analysis involves primary interviews, surveys, and vendor briefings. The data gathered as a result of these processes are validated through experts opinion. Our research methodology entails an ideal mixture of primary and secondary initiatives.
Frequently Asked Questions About This Report
PLACE AN ORDER
Year End Discount
Sample Report
Pre-Purchase Inquiry
NEED CUSTOMIZATION?
Request CustomizationCALL OR EMAIL US
100% Secure Payment






Related Reports
Our Global Clients
Our data-driven insights have influenced the strategy of 200+ reputed companies across the globe.