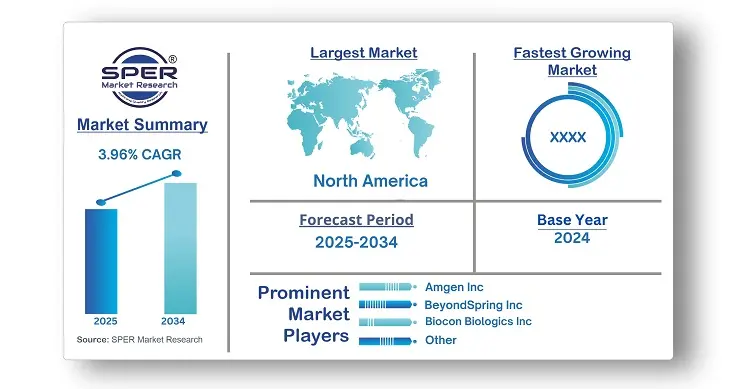
Chemotherapy-Induced Neutropenia Treatment Market Size 2034
Chemotherapy-Induced Neutropenia Treatment Market Growth, Size, Trends Analysis - By Treatment Type, By Drug Type, By Route of Administration, By Distribution Channel - Regional Outlook, Competitive Strategies and Segment Forecast to 2034
| Published: Oct-2025 | Report ID: HLCA25303 | Pages: 1 - 243 | Formats*: |
| Category : Healthcare | |||
| Report Metric | Details |
| Market size available for years | 2021-2034 |
| Base year considered | 2024 |
| Forecast period | 2025-2034 |
| Segments covered | By Treatment Type, By Drug Type, By Route of Administration, By Distribution Channel |
| Regions covered | North America, Latin America, Asia-Pacific, Europe, and Middle East & Africa |
| Companies Covered | Amgen Inc, BeyondSpring Inc, Biocon Biologics Inc, Cellerant Therapeutics, Coherus BioSciences, Inc, Evive Biotech, Kyowa Kirin Co., Ltd, Novartis AG, Pfizer Inc, Sanofi. |
- Global Chemotherapy-Induced Neutropenia Treatment Market Size (FY 2021-FY 2034)
- Overview of Global Chemotherapy-Induced Neutropenia Treatment Market
- Segmentation of Global Chemotherapy-Induced Neutropenia Treatment Market By Treatment Type (Granulocyte colony-stimulating factor therapy, Antibiotics, Granulocyte transfusion, Antifungals, Other treatment type)
- Segmentation of Global Chemotherapy-Induced Neutropenia Treatment Market By Drug Type (Branded, Biosimilars)
- Segmentation of Global Chemotherapy-Induced Neutropenia Treatment Market By Route of administration (Parenteral, Oral)
- Segmentation of Global Chemotherapy-Induced Neutropenia Treatment Market By Distribution Channel (Hospital pharmacies, Retail pharmacies, Online pharmacies)
- Statistical Snap of Global Chemotherapy-Induced Neutropenia Treatment Market
- Expansion Analysis of Global Chemotherapy-Induced Neutropenia Treatment Market
- Problems and Obstacles in Global Chemotherapy-Induced Neutropenia Treatment Market
- Competitive Landscape in the Global Chemotherapy-Induced Neutropenia Treatment Market
- Details on Current Investment in Global Chemotherapy-Induced Neutropenia Treatment Market
- Competitive Analysis of Global Chemotherapy-Induced Neutropenia Treatment Market
- Prominent Players in the Global Chemotherapy-Induced Neutropenia Treatment Market
- SWOT Analysis of Global Chemotherapy-Induced Neutropenia Treatment Market
- Global Chemotherapy-Induced Neutropenia Treatment Market Future Outlook and Projections (FY 2025-FY 2034)
- Recommendations from Analyst
- 1.1. Scope of the report
- 1.2. Market segment analysis
- 2.1. Research data source
- 2.1.1. Secondary Data
- 2.1.2. Primary Data
- 2.1.3. SPERs internal database
- 2.1.4. Premium insight from KOLs
- 2.2. Market size estimation
- 2.2.1. Top-down and Bottom-up approach
- 2.3. Data triangulation
- 4.1. Driver, Restraint, Opportunity and Challenges analysis
- 4.1.1. Drivers
- 4.1.2. Restraints
- 4.1.3. Opportunities
- 4.1.4. Challenges
- 5.1. SWOT Analysis
- 5.1.1. Strengths
- 5.1.2. Weaknesses
- 5.1.3. Opportunities
- 5.1.4. Threats
- 5.2. PESTEL Analysis
- 5.2.1. Political Landscape
- 5.2.2. Economic Landscape
- 5.2.3. Social Landscape
- 5.2.4. Technological Landscape
- 5.2.5. Environmental Landscape
- 5.2.6. Legal Landscape
- 5.3. PORTERs Five Forces
- 5.3.1. Bargaining power of suppliers
- 5.3.2. Bargaining power of buyers
- 5.3.3. Threat of Substitute
- 5.3.4. Threat of new entrant
- 5.3.5. Competitive rivalry
- 5.4. Heat Map Analysis
- 6.1. Global Chemotherapy-Induced Neutropenia Treatment Market Manufacturing Base Distribution, Sales Area, Product Type
- 6.2. Mergers & Acquisitions, Partnerships, Product Launch, and Collaboration in Global Chemotherapy-Induced Neutropenia Treatment Market
- 7.1. Granulocyte colony-stimulating factor therapy
- 7.2. Antibiotics
- 7.3. Granulocyte transfusion
- 7.4. Antifungals
- 7.5. Other treatment type
- 8.1. Branded
- 8.2. Biosimilars
- 9.1. Parenteral
- 9.2. Oral
- 10.1. Hospital pharmacies
- 10.2. Retail pharmacies
- 10.3. Online pharmacies
- 13.1. Global Chemotherapy-Induced Neutropenia Treatment Market Size and Market Share
- 12.1. Asia-Pacific
- 12.1.1. Australia
- 12.1.2. China
- 12.1.3. India
- 12.1.4. Japan
- 12.1.5. South Korea
- 12.1.6. Rest of Asia-Pacific
- 12.2. Europe
- 12.2.1. France
- 12.2.2. Germany
- 12.2.3. Italy
- 12.2.4. Spain
- 12.2.5. United Kingdom
- 12.2.6. Rest of Europe
- 12.3. Middle East and Africa
- 12.3.1. Kingdom of Saudi Arabia
- 12.3.2. United Arab Emirates
- 12.3.3. Qatar
- 12.3.4. South Africa
- 12.3.5. Egypt
- 12.3.6. Morocco
- 12.3.7. Nigeria
- 12.3.8. Rest of Middle-East and Africa
- 12.4. North America
- 12.4.1. Canada
- 12.4.2. Mexico
- 12.4.3. United States
- 12.5. Latin America
- 12.5.1. Argentina
- 12.5.2. Brazil
- 12.5.3. Rest of Latin America
- 13.1. Amgen Inc
- 13.1.1. Company details
- 13.1.2. Financial outlook
- 13.1.3. Product summary
- 13.1.4. Recent developments
- 13.2. BeyondSpring Inc
- 13.2.1. Company details
- 13.2.2. Financial outlook
- 13.2.3. Product summary
- 13.2.4. Recent developments
- 13.3. Biocon Biologics Inc
- 13.3.1. Company details
- 13.3.2. Financial outlook
- 13.3.3. Product summary
- 13.3.4. Recent developments
- 13.4. Cellerant Therapeutics
- 13.4.1. Company details
- 13.4.2. Financial outlook
- 13.4.3. Product summary
- 13.4.4. Recent developments
- 13.5. Coherus BioSciences, Inc
- 13.5.1. Company details
- 13.5.2. Financial outlook
- 13.5.3. Product summary
- 13.5.4. Recent developments
- 13.6. Evive Biotech
- 13.6.1. Company details
- 13.6.2. Financial outlook
- 13.6.3. Product summary
- 13.6.4. Recent developments
- 13.7. Kyowa Kirin Co., Ltd
- 13.7.1. Company details
- 13.7.2. Financial outlook
- 13.7.3. Product summary
- 13.7.4. Recent developments
- 13.8. Novartis AG
- 13.8.1. Company details
- 13.8.2. Financial outlook
- 13.8.3. Product summary
- 13.8.4. Recent developments
- 13.9. Pfizer Inc
- 13.9.1. Company details
- 13.9.2. Financial outlook
- 13.9.3. Product summary
- 13.9.4. Recent developments
- 13.10. Sanofi
- 15.10.1. Company details
- 15.10.2. Financial outlook
- 15.10.3 Product summary
- 15.10.4. Recent developments
- 13.11. Others
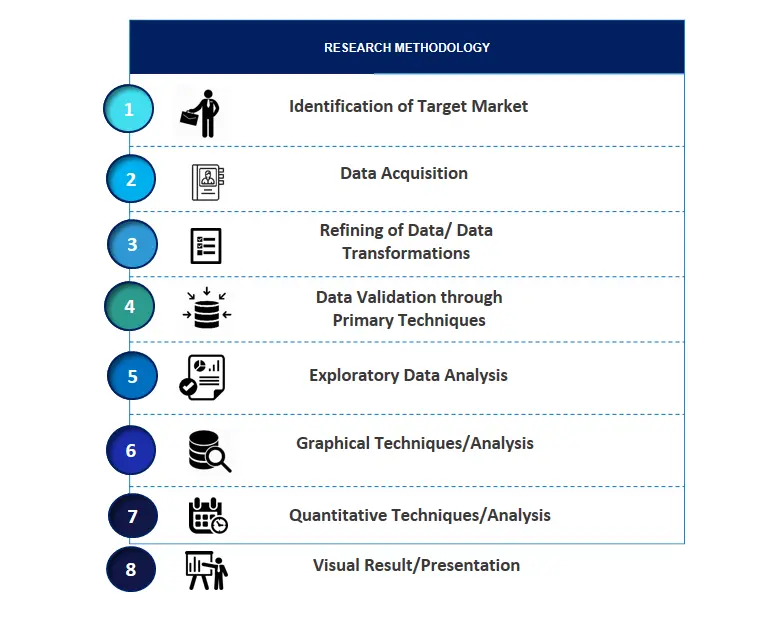
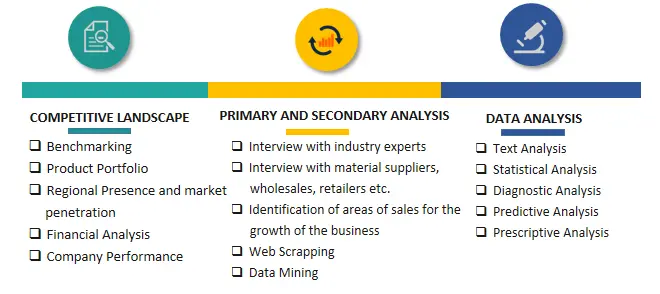
SPER Market Research’s methodology uses great emphasis on primary research to ensure that the market intelligence insights are up to date, reliable and accurate. Primary interviews are done with players involved in each phase of a supply chain to analyze the market forecasting. The secondary research method is used to help you fully understand how the future markets and the spending patterns look likes.
The report is based on in-depth qualitative and quantitative analysis of the Product Market. The quantitative analysis involves the application of various projection and sampling techniques. The qualitative analysis involves primary interviews, surveys, and vendor briefings. The data gathered as a result of these processes are validated through experts opinion. Our research methodology entails an ideal mixture of primary and secondary initiatives.
Frequently Asked Questions About This Report
PLACE AN ORDER
Year End Discount
Sample Report
Pre-Purchase Inquiry
NEED CUSTOMIZATION?
Request CustomizationCALL OR EMAIL US
100% Secure Payment






Related Reports
Our Global Clients
Our data-driven insights have influenced the strategy of 200+ reputed companies across the globe.