Congestive Heart Failure Treatment Devices Market Introduction and Overview
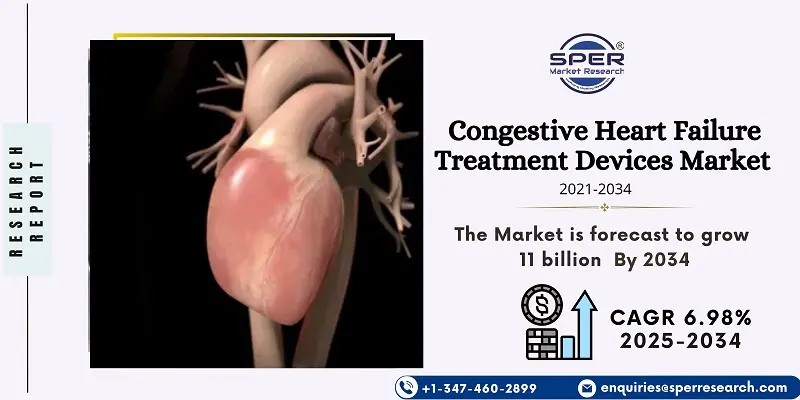
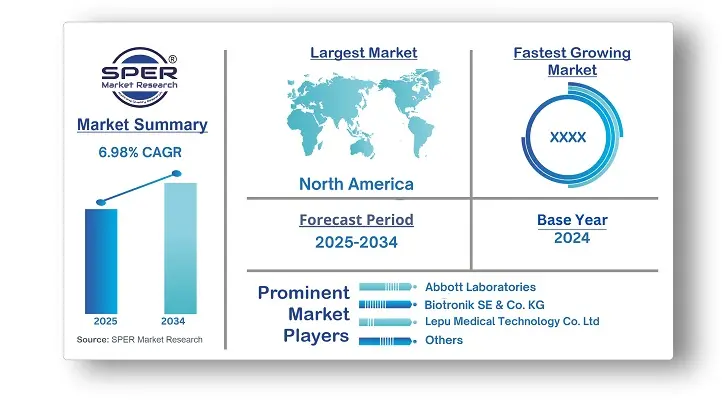
According to SPER Market Research, the Global Congestive Heart Failure Treatment Devices Market is estimated to reach USD 11 billion by 2034 with a CAGR of 6.98%.
The report includes an in-depth analysis of the Global Congestive Heart Failure Treatment Devices Market, including market size and trends, product mix, Applications, and supplier analysis. The increasing prevalence of heart failure and the demand for cutting-edge treatment options are driving growth in the market for congestive heart failure (CHF) treatment devices. Devices that improve cardiac function and patient survival, such as pacemakers, ventricular assist devices (VADs), implanted Cardioverter defibrillators (ICDs), and cardiac resynchronization treatment (CRT) devices, are essential for managing congestive heart failure (CHF). The prevalence of cardiovascular diseases is on the rise, technology is developing, and early intervention knowledge is growing. Nevertheless, obstacles like exorbitant device expenses, rigorous regulatory clearances, and possible implantation process issues impede market expansion. Industry participants continue to prioritize overcoming these obstacles via innovation and increased affordability.
By Product Insights
Congestive Heart Failure Treatment Devices is classified into five categories based on Product: Ventricular assist devices, Counter Pulsation Devices, Implantable Cardioverter Defibrillators, Pacemakers, Cardiac Resynchronization therapy. The segment with the largest revenue share was implanted Cardioverter defibrillators, or ICDs. Because of their vital function in preventing sudden cardiac arrest in patients with arrhythmias, implantable Cardioverter defibrillators, or ICDs, dominate the cardiac device industry. The growing number of older people with chronic illnesses such diabetes, stroke, heart disease, neurological diseases, and hypertension is expected to drive the fastest compound annual growth rate (CAGR) in the ventricular assist devices market.
By Regional Insights
The global market for congestive heart failure therapy devices was led by North America. An aging population, a high rate of heart disease, and sophisticated healthcare infrastructure are the main causes of this. Continuous research and technical developments in the medical industry also contribute to the increasing use of cutting-edge treatment tools like heart pumps and ventricular assist devices (VADs).
Market Competitive Landscape
Leading businesses in the sector aggressively pursue a range of strategic goals to strengthen their position in the market. Investing in R&D to innovate and improve the qualities of congestive heart failure therapy devices is one of these activities, which aims to improve clinical outcomes and improve patient comfort. Some of the key market players are Abbott Laboratories, Biotronik SE & Co. KG, Boston Scientific Corporation, Koninkilijke Philips N.V., Lepu Medical Technology Co. Ltd., LivaNova PLC, MEDICO S.p.A., Medtronic plc, MicroPort Scientific Corporation, OSCOR Inc.
Recent Developments:
In December 2024, The FDA has broadened the indications for the Impella 5.5 with SmartAssist and Impella CP with SmartAssist heart pumps, according to a statement from Johnson & Johnson MedTech. An important step forward in the treatment choices for critically ill children has been made possible by this premarket approval, which permits their use in specific pediatric patients with symptomatic acute decompensated heart failure and cardiogenic shock.
In September 2024, The FDA authorized Boston Scientific Corporation's expansion of the use of its INGEVITY+ Pacing Leads for conduction system pacing and left bundle branch region monitoring with single- or dual-chamber pacemakers. This technique reduces the long-term risk of heart failure and may improve ventricular synchronization when used in place of conventional right ventricular pacing to treat symptomatic bradycardia.
Scope of the report:
| Report Metric | Details |
| Market size available for years | 2021-2034 |
| Base year considered | 2024 |
| Forecast period | 2025-2034 |
| Segments covered | By Product |
| Regions covered | North America, Latin America, Asia-Pacific, Europe, and Middle East & Africa |
| Companies Covered | Abbott Laboratories, Biotronik SE & Co. KG, Boston Scientific Corporation, Koninkilijke Philips N.V., Lepu Medical Technology Co. Ltd., LivaNova PLC, MEDICO S.p.A., Medtronic plc, MicroPort Scientific Corporation, OSCOR Inc.
|
Key Topics Covered in the Report
- Global Congestive Heart Failure Treatment Devices Market Size (FY’2021-FY’2034)
- Overview of Global Congestive Heart Failure Treatment Devices Market
- Segmentation of Global Congestive Heart Failure Treatment Devices Market By Product (Ventricular assist devices, Counter Pulsation Devices, Implantable Cardioverter Defibrillators, Pacemakers, Cardiac Resynchronization therapy)
- Statistical Snap of Global Congestive Heart Failure Treatment Devices Market
- Expansion Analysis of Global Congestive Heart Failure Treatment Devices Market
- Problems and Obstacles in Global Congestive Heart Failure Treatment Devices Market
- Competitive Landscape in the Global Congestive Heart Failure Treatment Devices Market
- Details on Current Investment in Global Congestive Heart Failure Treatment Devices Market
- Competitive Analysis of Global Congestive Heart Failure Treatment Devices Market
- Prominent Players in the Global Congestive Heart Failure Treatment Devices Market
- SWOT Analysis of Global Congestive Heart Failure Treatment Devices Market
- Global Congestive Heart Failure Treatment Devices Market Future Outlook and Projections (FY’2025-FY’2034)
- Recommendations from Analyst
1. Introduction
1.1. Scope of the report
1.2. Market segment analysis
2. Research Methodology
2.1. Research data source
2.1.1. Secondary Data
2.1.2. Primary Data
2.1.3. SPER’s internal database
2.1.4. Premium insight from KOL’s
2.2. Market size estimation
2.2.1. Top-down and Bottom-up approach
2.3. Data triangulation
3. Executive Summary
4. Market Dynamics
4.1. Driver, Restraint, Opportunity and Challenges analysis
4.1.1. Drivers
4.1.2. Restraints
4.1.3. Opportunities
4.1.4. Challenges
5. Market variable and outlook
5.1. SWOT Analysis
5.1.1. Strengths
5.1.2. Weaknesses
5.1.3. Opportunities
5.1.4. Threats
5.2. PESTEL Analysis
5.2.1. Political Landscape
5.2.2. Economic Landscape
5.2.3. Social Landscape
5.2.4. Technological Landscape
5.2.5. Environmental Landscape
5.2.6. Legal Landscape
5.3. PORTER’s Five Forces
5.3.1. Bargaining power of suppliers
5.3.2. Bargaining power of buyers
5.3.3. Threat of Substitute
5.3.4. Threat of new entrant
5.3.5. Competitive rivalry
5.4. Heat Map Analysis
6. Competitive Landscape
6.1. Global Congestive Heart Failure Treatment Devices Market Manufacturing Base Distribution, Sales Area, Product Type
6.2. Mergers & Acquisitions, Partnerships, Product Launch, and Collaboration in Global Congestive Heart Failure Treatment Devices Market
7. Global Congestive Heart Failure Treatment Devices Market, By Product 2021-2034 (USD Million)
7.1. Ventricular assist devices
7.1.1. LVAD
7.1.2. RVAD
7.1.3. BiVAD
7.2. Counter Pulsation Devices
7.3. Implantable Cardioverter Defibrillators
7.3.1. Transvenous ICDs
7.3.2. Subcutaneous ICDs
7.4. Pacemakers
7.4.1. Implantable
7.4.2. External
7.5. Cardiac Resynchronization therapy
7.5.1. Cardiac Resynchronization Therapy-Defibrillators (CRT-D)
7.5.2. Cardiac Resynchronization Therapy-Pacemakers (CRT-P)
8. Global Congestive Heart Failure Treatment Devices Market, 2021-2034 (USD Million)
8.1. Global Congestive Heart Failure Treatment Devices Market Size and Market Share
9. Global Congestive Heart Failure Treatment Devices Market, By Region, 2021-2034 (USD Million)
9.1. Asia-Pacific
9.1.1. Australia
9.1.2. China
9.1.3. India
9.1.4. Japan
9.1.5. South Korea
9.1.6. Rest of Asia-Pacific
9.2. Europe
9.2.1. France
9.2.2. Germany
9.2.3. Italy
9.2.4. Spain
9.2.5. United Kingdom
9.2.6. Rest of Europe
9.3. Middle East and Africa
9.3.1. Kingdom of Saudi Arabia
9.3.2. United Arab Emirates
9.3.3. Qatar
9.3.4. South Africa
9.3.5. Egypt
9.3.6. Morocco
9.3.7. Nigeria
9.3.8. Rest of Middle-East and Africa
9.4. North America
9.4.1. Canada
9.4.2. Mexico
9.4.3. United States
9.5. Latin America
9.5.1. Argentina
9.5.2. Brazil
9.5.3. Rest of Latin America
10. Company Profile
10.1. Abbott Laboratories
10.1.1. Company details
10.1.2. Financial outlook
10.1.3. Product summary
10.1.4. Recent developments
10.2. Biotronik SE & Co. KG
10.2.1. Company details
10.2.2. Financial outlook
10.2.3. Product summary
10.2.4. Recent developments
10.3. Boston Scientific Corporation
10.3.1. Company details
10.3.2. Financial outlook
10.3.3. Product summary
10.3.4. Recent developments
10.4. Koninkilijke Philips N.V.
10.4.1. Company details
10.4.2. Financial outlook
10.4.3. Product summary
10.4.4. Recent developments
10.5. Lepu Medical Technology Co. Ltd.
10.5.1. Company details
10.5.2. Financial outlook
10.5.3. Product summary
10.5.4. Recent developments
10.6. LivaNova PLC
10.6.1. Company details
10.6.2. Financial outlook
10.6.3. Product summary
10.6.4. Recent developments
10.7. MEDICO S.p.A.
10.7.1. Company details
10.7.2. Financial outlook
10.7.3. Product summary
10.7.4. Recent developments
10.8. Medtronic plc
10.8.1. Company details
10.8.2. Financial outlook
10.8.3. Product summary
10.8.4. Recent developments
10.9. MicroPort Scientific Corporation
10.9.1. Company details
10.9.2. Financial outlook
10.9.3. Product summary
10.9.4. Recent developments
10.10. OSCOR Inc.
10.10.1. Company details
10.10.2. Financial outlook
10.10.3. Product summary
10.10.4. Recent developments
11.11. Others
11. Conclusion
12. List of Abbreviations
13. Reference Links