
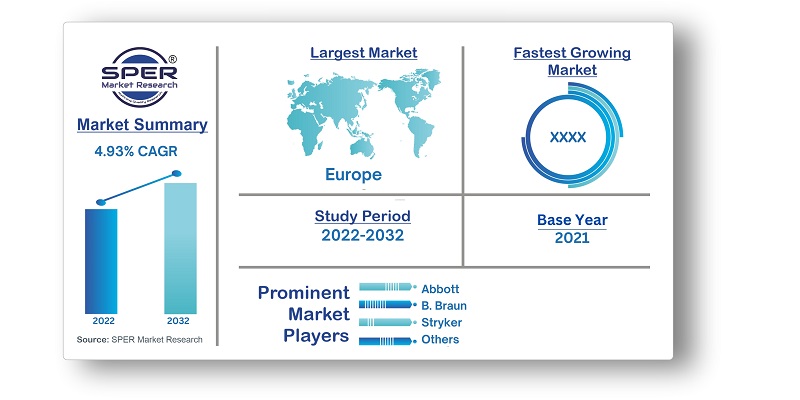
Europe Medical Device Market Growth, Trends, Size, Revenue, Scope, Challenges and Future Outlook
Europe Medical Device Market Size- By Device Type, By End User- Regional Outlook, Competitive Strategies and Segment Forecast to 2032
| Published: Mar-2023 | Report ID: MEDE2311 | Pages: 1 - 159 | Formats*: |
| Category : Medical Devices | |||
- April 2022: Getinge AB signed a contract to buy all of the stock in FLUOPTICS SAS, a French firm that specialises in fluorescence imaging to help with surgery. With safe and enhanced operating efficiency, the acquisition sought to increase Getinge's client offering in clinical decision assistance.
- April 2022: The reimbursement for MiniMed 780G, a sophisticated insulin delivery device, has been expanded to two more European nations, including France and Germany, according to a statement from Medtronic plc.
- Opportunities: Most medical device businesses are going through a big technology transition that will eventually change them into MedTech firms. One of the most inventive and diversified industries in the world, MedTech is expected to expand due to expanding trade flows, an increase in the number of patent applications made by the sector, a high rate of employment and retention, and improvements to the European healthcare system. In an effort to be closer to the end user, manufacturers are harnessing data to infuse intelligence into their devices, and it is quickly becoming into a critical part of the new device value proposition. Additionally, organisations are taking part in atypical coalitions. Artificial intelligence developments in the future will make it easier for patients to manage chronic conditions. A major impact of technology will be seen on both prevention and treatment, which will result in less time spent in hospitals. For instance, industrial companies are placing more and more emphasis on robotic surgery.
- Challenges: Despite increased innovation and the introduction of new products, European medical practitioners are compelled to employ traditional practises. Medical technology now includes artificial intelligence significantly. Its clearance criteria are still unclear, and prohibiting medical personnel from adopting cutting-edge items causes delays and restricts patient access to healthcare.

| Report Metric | Details |
| Market size available for years | 2019-2032 |
| Base year considered | 2021 |
| Forecast period | 2022-2032 |
| Segments covered | By Device Type, By End User. |
| Regions covered | Germany, UK, France, Italy, Spain, Poland, Rest of Europe |
| Companies Covered | Abbott, B. Braun, Boston Scientific, Fujifilm, GE Healthcare, Karl Storz, Masimo, Medtronic, Olympus, Philips, ResMed, Siemens Healthineers, Stryker |
- Distributors and Suppliers
- Healthcare Institutions
- Healthcare IT Providers
- Healthcare Professionals
- Medical Device Manufacturers
- Patients and Caregivers
- Patients and Patient Advocacy Groups
- Others
| By Device Type: |
|
| By End User: |
|
- Size of Europe Medical Device Market (FY’2019-FY’2032)
- Overview of Europe Medical Device Market
- Segmentation of Europe Medical Device Market By Device Type (Imaging System, Endoscopy Device, Interventional Cardiology Devices, Cardiac Monitoring and Cardiac Rhythm Management Devices, Ventilators, Orthopedic Devices, Ophthalmic Devices, Diabetes Care Devices, Dialysis Devices, Anesthesia Monitoring, Respiratory Care Devices)
- Segmentation of Europe Medical Device Market By End User (Hospital and Clinic Care Settings, Home-care Settings)
- Statistical Snap of Europe Medical Device Market
- Europe Medical Device Market Growth Analysis
- Problems and Challenges in Europe Medical Device Market
- Europe Medical Device Market Competitive Landscape
- Impact of COVID-19 and Demonetization on Europe Medical Device Market
- Details on Recent Investment in Europe Medical Device Market
- Competitive Analysis of Europe Medical Device Market
- Major Players in the Europe Medical Device Market
- SWOT Analysis of Europe Medical Device Market
- Europe Medical Device Market Future Outlook and Projections (FY’2019-FY’2032)
- Recommendations from Analyst
1.1. Scope of the report1.2. Market segment analysis
2.1 Research data source2.1.1 Secondary data2.1.2 Primary data2.1.3 SPER’s internal database2.1.4 Premium insight from KOL’s2.2 Market size estimation2.2.1 Top-down and Bottom-up approach2.3 Data triangulation
4.1. Driver, Restraint, Opportunity and Challenges analysis4.1.1 Drivers4.1.2 Restraints4.1.3 Opportunities4.1.4 Challenges4.2. COVID-19 Impacts of the Europe Medical Device Market
5.1. SWOT analysis5.1.1 Strengths5.1.2 Weaknesses5.1.3 Opportunities5.1.4 Threats5.2. PESTEL analysis5.2.1 Political landscape5.2.2 Economic landscape5.2.3 Social landscape5.2.4 Technological landscape5.2.5 Environmental landscape5.2.6 Legal landscape5.3. PORTER’S five forces analysis5.3.1 Bargaining power of suppliers5.3.2 Bargaining power of Buyers5.3.3 Threat of Substitute5.3.4 Threat of new entrant5.3.5 Competitive rivalry5.4. Heat map analysis
6.1 Europe Medical Device Manufacturing Base Distribution, Sales Area, Product Type6.2 Mergers & Acquisitions, Partnerships, Product Launch, and Collaboration in Europe Medical Device Market
7.1 Imaging System7.2 Endoscopy Device7.3 Interventional Cardiology Devices7.4 Cardiac Monitoring and Cardiac Rhythm Management Devices7.5 Ventilators7.6 Orthopedic Devices7.7 Ophthalmic Devices7.8 Diabetes Care Devices7.9 Dialysis Devices7.10 Anesthesia Monitoring7.11 Respiratory Care Devices
8.1 Hospital and Clinic Care Settings8.2 Home-care Settings
9.1 Europe Medical Device Size and Market Share by Region (2019-2025)9.2 Europe Medical Device Size and Market Share by Region (2026-2032)9.3 Germany9.4 UK9.5 France9.6 Italy9.7 Spain9.8 Poland9.9 Rest of Europe
10.1 Abbott10.1.1 Company details10.1.2 Financial outlook10.1.3 Product summary10.1.4 Recent developments10.2 B. Braun10.2.1 Company details10.2.2 Financial outlook10.2.3 Product summary10.2.4 Recent developments10.3 Boston Scientific10.3.1 Company details10.3.2 Financial outlook10.3.3 Product summary10.3.4 Recent developments10.4 Fujifilm10.4.1 Company details10.4.2 Financial outlook10.4.3 Product summary10.4.4 Recent developments10.5 GE Healthcare10.5.1 Company details10.5.2 Financial outlook10.5.3 Product summary10.5.4 Recent developments10.6 Karl Storz10.6.1 Company details10.6.2 Financial outlook10.6.3 Product summary10.6.4 Recent developments10.7 Masimo10.7.1 Company details10.7.2 Financial outlook10.7.3 Product summary10.7.4 Recent developments10.8 Medtronic10.8.1 Company details10.8.2 Financial outlook10.8.3 Product summary10.8.4 Recent developments10.9 Olympus10.9.1 Company details10.9.2 Financial outlook10.9.3 Product summary10.9.4 Recent developments10.10 Philips10.10.1 Company details10.10.2 Financial outlook10.10.3 Product summary10.10.4 Recent developments10.11 ResMed10.11.1 Company details10.11.2 Financial outlook10.11.3 Product summary10.11.4 Recent developments10.12 Siemens Healthieers10.12.1 Company details10.12.2 Financial outlook10.12.3 Product summary10.12.4 Recent developments10.13 Stryker10.13.1 Company details10.13.2 Financial outlook10.13.3 Product summary10.13.4 Recent developments
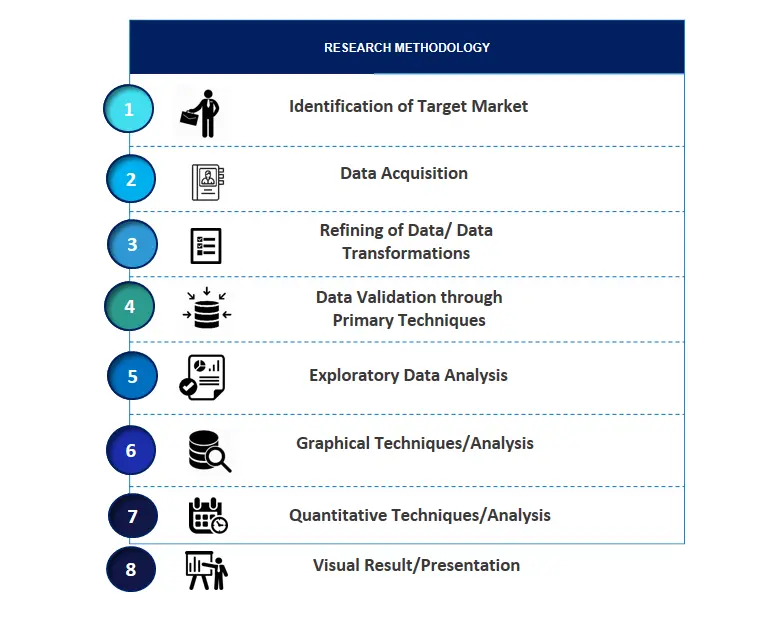
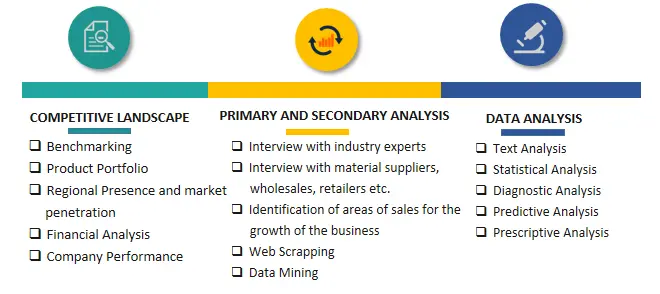
SPER Market Research’s methodology uses great emphasis on primary research to ensure that the market intelligence insights are up to date, reliable and accurate. Primary interviews are done with players involved in each phase of a supply chain to analyze the market forecasting. The secondary research method is used to help you fully understand how the future markets and the spending patterns look likes.
The report is based on in-depth qualitative and quantitative analysis of the Product Market. The quantitative analysis involves the application of various projection and sampling techniques. The qualitative analysis involves primary interviews, surveys, and vendor briefings. The data gathered as a result of these processes are validated through experts opinion. Our research methodology entails an ideal mixture of primary and secondary initiatives.
Frequently Asked Questions About This Report
PLACE AN ORDER
Year End Discount
Sample Report
Pre-Purchase Inquiry
NEED CUSTOMIZATION?
Request CustomizationCALL OR EMAIL US
100% Secure Payment






Related Reports
Our Global Clients
Our data-driven insights have influenced the strategy of 200+ reputed companies across the globe.






















