IVD In Cardiology and Neurology Market Introduction and Overview
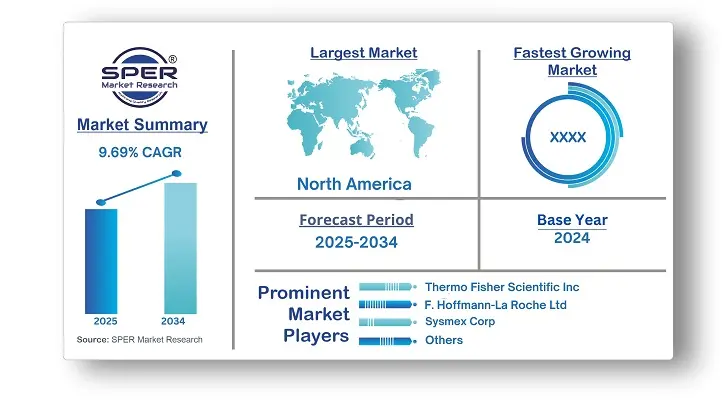
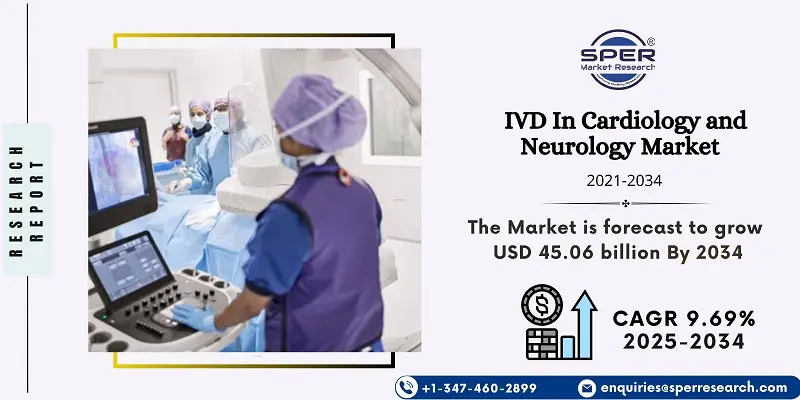
According to SPER Market Research, the Global IVD In Cardiology and Neurology Market is estimated to reach USD 45.06 billion by 2034 with a CAGR of 9.69%.
The report includes an in-depth analysis of the Global IVD In Cardiology and Neurology Market, including market size and trends, product mix, Applications, and supplier analysis. The global IVD market for cardiology and neurology was valued at USD 17.87 billion in 2024 and is expected to expand at a compound annual growth rate (CAGR) of 9.69% between 2025 and 2034. The increasing incidence of neurological and cardiovascular conditions, improvements in clinical diagnostic technology, and a growing emphasis on research to create new diagnostics for the market are all factors contributing to the industry's expansion. Growth is also being accelerated by rising investments from both public and private entities.
By Product Type Insights
The reagents and consumables segment led the in vitro diagnostics (IVD) market in cardiology and neurology, accounting for the largest revenue in 2024 and expected to grow fastest from 2025 to 2034. Reagents like fluorescent tags, washing buffers, specific antibodies, and protein biomarkers are used in immunochemistry assays. Chemicals like dNTP mix, primer mix, and magnesium chloride are for PCR tests. Key players in providing reagents include Abbott, Illumina, Inc. , and Roche Diagnostics. Growth is due to significant R&D by major players for new biomarker kits and the introduction of new reagents.
By Technology Insights
In 2024, the immunoassays sector held the largest market share. Immunology studies how the immune system works, focusing on antibodies, antigens, and their interactions. Companies are working on developing and selling immunoassays.
The molecular diagnostics segment is expected to grow the fastest from 2025 to 2034. These technologies help identify gene changes linked to cardiovascular diseases. The various techniques in molecular diagnostics can find mutations related to cardiovascular and neurological disorders, offering growth opportunities for immunoassays. Industry players are also focusing on collaborations and new product developments to expand their reach.
By End-User Insights
In 2024, the hospital industry had the largest market share. The segment's growth is due to an increase in hospitalisation, as doctors require diagnostic interpretation for subsequent treatment. Most diagnostic centres work in partnership with hospitals, thus hospitals have their own diagnostic setup. Furthermore, continued expansion of healthcare infrastructure is expected to improve existing hospital facilities. As a result, the need for hospital-based IVD tests is growing. The majority of IVD devices are acquired by hospitals and used in large numbers.
Regional Insights
North America accounted for the largest share in 2024 due to factors like the increasing burden of neurological and cardiovascular disorders and more favorable government initiatives. Rising product launches and funding for research and development of novel biomarkers are expected to boost market growth. The greater use of IVDs in diagnosing acute coronary syndrome is increasing regional demand. Unhealthy lifestyles are also raising the rates of acute coronary syndrome and myocardial infarction. Additionally, the widespread adoption of point-of-care diagnostics is fostering regional innovation, making in vitro diagnostics more accessible.
Market Competitive Landscape
The IVD in cardiology and neurology industry is competitive, with large multinationals and smaller companies in the market. A key strategy is developing and launching new advanced products and services using different technologies. Leading players include Siemens Healthineers AG, Abbott, and Beckman Coulter, Inc. These companies focus on maintaining high-quality standards and expanding their market access by leveraging existing customer bases. They invest heavily in advanced technology and infrastructure for efficient sample processing and analysis. Additionally, companies pursue strategic partnerships with other firms and distributors to enhance their market presence.
Recent Developments:
The U.S. FDA approved Abbott's first commercially accessible laboratory traumatic brain injury (TBI) blood test in March 2023. This test, which was based on Abbott's Alinity i laboratory technology, revolutionised the company's product line by giving clinicians an objective method to rapidly evaluate patients with moderate TBIs.
In March 2023, Fujirebio announced the introduction of the fully automated Lumipulse G NfL Blood and Lumipulse G NfL CSF assays, which would broaden its assay product offering in the area of neurodegenerative illnesses.
Scope of the report:
| Report Metric | Details |
| Market size available for years | 2021-2034 |
| Base year considered | 2024 |
| Forecast period | 2025-2034 |
| Segments covered | By Product Type, By Technology, By End-User |
| Regions covered | North America, Latin America, Asia-Pacific, Europe, and Middle East & Africa |
| Companies Covered | Thermo Fisher Scientific Inc, F. Hoffmann-La Roche Ltd, Sysmex Corp, Siemens Healthineers AG, Quest Diagnostics Inc, Abbott, BD, Bio-Rad Laboratories, Inc, Beckman Coulter, Inc.
|
Key Topics Covered in the Report
- Global IVD In Cardiology and Neurology Market Size (FY’2021-FY’2034)
- Overview of Global IVD In Cardiology and Neurology Market
- Segmentation of Global IVD In Cardiology and Neurology Market By Product Type (Instruments, Reagents & Consumables, Software and Services)
- Segmentation of Global IVD In Cardiology and Neurology Market By Technology (Immunoassays, Molecular Diagnostics, Hematology, Others)
- Segmentation of Global IVD In Cardiology and Neurology Market By End-User (Hospitals, Clinical Laboratories, Others)
- Statistical Snap of Global IVD In Cardiology and Neurology Market
- Expansion Analysis of Global IVD In Cardiology and Neurology Market
- Problems and Obstacles in Global IVD In Cardiology and Neurology Market
- Competitive Landscape in the Global IVD In Cardiology and Neurology Market
- Details on Current Investment in Global IVD In Cardiology and Neurology Market
- Competitive Analysis of Global IVD In Cardiology and Neurology Market
- Prominent Players in the Global IVD In Cardiology and Neurology Market
- SWOT Analysis of Global IVD In Cardiology and Neurology Market
- Global IVD In Cardiology and Neurology Market Future Outlook and Projections (FY’2025-FY’2034)
- Recommendations from Analyst
1. Introduction
1.1. Scope of the report
1.2. Market segment analysis
2. Research Methodology
2.1. Research data source
2.1.1. Secondary Data
2.1.2. Primary Data
2.1.3. SPER’s internal database
2.1.4. Premium insight from KOL’s
2.2. Market size estimation
2.2.1. Top-down and Bottom-up approach
2.3. Data triangulation
3. Executive Summary
4. Market Dynamics
4.1. Driver, Restraint, Opportunity and Challenges analysis
4.1.1. Drivers
4.1.2. Restraints
4.1.3. Opportunities
4.1.4. Challenges
5. Market variable and outlook
5.1. SWOT Analysis
5.1.1. Strengths
5.1.2. Weaknesses
5.1.3. Opportunities
5.1.4. Threats
5.2. PESTEL Analysis
5.2.1. Political Landscape
5.2.2. Economic Landscape
5.2.3. Social Landscape
5.2.4. Technological Landscape
5.2.5. Environmental Landscape
5.2.6. Legal Landscape
5.3. PORTER’s Five Forces
5.3.1. Bargaining power of suppliers
5.3.2. Bargaining power of buyers
5.3.3. Threat of Substitute
5.3.4. Threat of new entrant
5.3.5. Competitive rivalry
5.4. Heat Map Analysis
6. Competitive Landscape
6.1. Global IVD In Cardiology and Neurology Market Manufacturing Base Distribution, Sales Area, Product Type
6.2. Mergers & Acquisitions, Partnerships, Product Launch, and Collaboration in Global IVD In Cardiology and Neurology Market
7. Global IVD In Cardiology and Neurology Market, By Product Type (USD Million) 2021-2034
7.1. Instruments
7.2. Reagents & Consumables
7.3. Software and Services
8. Global IVD In Cardiology and Neurology Market, By Technology (USD Million) 2021-2034
8.1. Immunoassays
8.2. Molecular Diagnostics
8.3. Hematology
8.4. Others
9. Global IVD In Cardiology and Neurology Market, By End-User (USD Million) 2021-2034
9.1. Hospitals
9.2. Clinical Laboratories
9.3. Others
10. Global IVD In Cardiology and Neurology Market, (USD Million) 2021-2034
10.1. Global IVD In Cardiology and Neurology Market Size and Market Share
11. Global IVD In Cardiology and Neurology Market, By Region, (USD Million) 2021-2034
11.1. Asia-Pacific
11.1.1. Australia
11.1.2. China
11.1.3. India
11.1.4. Japan
11.1.5. South Korea
11.1.6. Rest of Asia-Pacific
11.2. Europe
11.2.1. France
11.2.2. Germany
11.2.3. Italy
11.2.4. Spain
11.2.5. United Kingdom
11.2.6. Rest of Europe
11.3. Middle East and Africa
11.3.1. Kingdom of Saudi Arabia
11.3.2. United Arab Emirates
11.3.3. Qatar
11.3.4. South Africa
11.3.5. Egypt
11.3.6. Morocco
11.3.7. Nigeria
11.3.8. Rest of Middle-East and Africa
11.4. North America
11.4.1. Canada
11.4.2. Mexico
11.4.3. United States
11.5. Latin America
11.5.1. Argentina
11.5.2. Brazil
11.5.3. Rest of Latin America
12. Company Profile
12.1. Thermo Fisher Scientific Inc
12.1.1. Company details
12.1.2. Financial outlook
12.1.3. Product summary
12.1.4. Recent developments
12.2. F. Hoffmann-La Roche Ltd
12.2.1. Company details
12.2.2. Financial outlook
12.2.3. Product summary
12.2.4. Recent developments
12.3. Sysmex Corporation
12.3.1. Company details
12.3.2. Financial outlook
12.3.3. Product summary
12.3.4. Recent developments
12.4. Siemens Healthineers AG
12.4.1. Company details
12.4.2. Financial outlook
12.4.3. Product summary
12.4.4. Recent developments
12.5. Quest Diagnostics Incorporated
12.5.1. Company details
12.5.2. Financial outlook
12.5.3. Product summary
12.5.4. Recent developments
12.6. Abbott
12.6.1. Company details
12.6.2. Financial outlook
12.6.3. Product summary
12.6.4. Recent developments
12.7. BD
12.7.1. Company details
12.7.2. Financial outlook
12.7.3. Product summary
12.7.4. Recent developments
12.8. Bio-Rad Laboratories, Inc
12.8.1. Company details
12.8.2. Financial outlook
12.8.3. Product summary
12.8.4. Recent developments
12.9. Beckman Coulter, Inc
12.9.1. Company details
12.9.2. Financial outlook
12.9.3. Product summary
12.9.4. Recent developments
12.10. Others
13. Conclusion
14. List of Abbreviations
15. Reference Links